Cryopreserving Coral Sperm for Reef Conservation & Restoration (Part 2)
For an introduction to cryopreservation and its importance in corals, go check out Part 1 of this article series.
Ph.D. student Liv Williamson collects gamete bundles from Montipora flabellata. Once the bundles separate into eggs and sperm, the research team will cryopreserve the sperm.
Progress in corals
While cryopreservation techniques have been used for decades in threatened terrestrial animals such as rhinos (and also in humans), they are only starting to be applied to coral conservation. Studies by Dr. Mary Hagedorn and colleagues have pioneered the use of cryopreservation for coral spermatozoa. To date, her team has successfully frozen ~30 species of corals, primarily from Hawai’i and the Great Barrier Reef, and frozen sperm from these species are being stored long-term with the USDA’s Animal Germplasm Program (Fort Collins, CO, USA).
In 2012, Hagedorn and colleagues published the results of a study that generated Acropora tenuis and Acropora millepora larvae from frozen sperm, the first known confirmation that cryopreserved coral sperm could be thawed and remain reproductively viable. They have since shown no difference in fertilization success depending on the length of time that sperm has been frozen (ranging from a few hours to 2 years), illustrating the promise of this approach for long-term storage of viable sperm for use in conservation efforts (Hagedorn et al. 2017).
Hagedorn and her colleagues are beginning to publish more on their sperm freezing and thawing techniques to disseminate the knowledge to scientists and restoration practitioners around the world, in hopes that anyone with access to coral spawning events can incorporate cryopreservation into their efforts.
Dr. Mary Hagedorn and Dr. Virginia Carter are two pioneers in the study of coral cryobiology. Photo credit: J Daniels, Smithsonian Global
Recently, Hagedorn and colleagues demonstrated the potential for gametes from distant populations of the same species to successfully fertilize one another after cryopreservation and result in viable, settled offspring (Hagedorn et al. 2018). Using the threatened Caribbean species Acropora palmata (staghorn coral), this study succeeded in fertilizing fresh eggs from Curaçao with frozen sperm from Curaçao, Florida, and Puerto Rico, and all crosses resulted in swimming larvae and settled recruits. These results demonstrated that (1) distant conspecific populations can remain reproductively compatible, and (2) genetic diversity can be transferred from one population to another for the purposes of assisted gene flow in corals (Hagedorn et al. 2018)!
Endangered Acropora palmata, the elkhorn coral, is an important reef-building species in the Caribbean. Sperm from this species has been successfully cryopreserved and used to selectively breed offspring. Photo credit: Liv Williamson
Unfertilized eggs (smooth, round) and fertilized embryos (textured, bumpy) of Orbicella faveolata (mountainous star coral) under a microscope. Photo credit: Liv Williamson
Limitations and knowledge gaps
Despite these promising applications, coral cryopreservation has a number of limitations and remaining knowledge gaps. First, freezing sperm almost always decreases its motility, often by about 50% (Hagedorn et al. 2012). For instance, fresh sperm with 90% motility prior to freezing will likely have ~45% motility after it is thawed. Therefore, it is essential to obtain robust, highly motile sperm to use for freezing (Hagedorn et al. 2017), because high motility yields better fertilization.
Furthermore, a portion of sperm cells will die as a result of the freezing and thawing, which decreases the concentration of sperm and thus decreases the rate of fertilization it is likely to achieve. The process of freezing and thawing any biological material is fraught with risk, as forming ice crystals threaten to cause lethal damage and changing concentrations of water and salts threaten to disrupt the structure and/or function of proteins, organelles, cells, and tissues (Hagedorn et al. 2006, Benson et al. 2012). As such, it is important to maintain a relatively high sperm-to-egg ratio when attempting to fertilize fresh eggs with cryopreserved sperm (Hagedorn et al. 2017).
Lobactis scutaria female releasing her eggs. This species is gonochoric, meaning individuals are either male or female and produce either sperm or eggs. Photo credit: Liv Williamson
Another limitation is the failure to successfully freeze and thaw coral eggs. With a larger size and much more complex structure than sperm cells, eggs are more susceptible to damage during the extreme temperature changes. To date, no one has been able to thaw coral eggs without ice crystals rupturing and killing them during some part of the process.
If we can only freeze sperm, we cannot freeze gametes from female colonies. Although the majority of corals are hermaphrodites, many species are gonochoric, producing either eggs or sperm (Shlesinger et al. 1998). Without female gametes, we lack the genetic material from female corals, and thus cannot capture the full scope of diversity in gonochoric populations. In addition, our ability to artificially aid in reproduction and selectively breed corals is restricted without cryopreserved eggs. Using frozen sperm, we are still only able to create offspring when fresh eggs become available, which only happens on a few nights a year for any given species.
One possible solution to increase the availability of fresh eggs is spawning corals in captivity. The Horniman Museum in London and the Florida Aquarium have tackled this challenge with facilities that mimic environmental conditions to artificially induce spawning in corals at various times throughout the year, enabling captive coral spawning research. Inducing corals to spawn at different times during the year can minimize the challenges of narrow reproductive timing in corals, and thus increase the amount of sperm available for freezing and/or fresh eggs for fertilizing.
Lobactis scutaria female releasing her eggs. Photo credit: Liv Williamson
Next steps
Sexual reproduction in wild corals seems to be failing for a variety of reasons. A lack of sexual reproduction means a devastating dearth of new genetic diversity and new recruits reaching reefs that desperately need them to help with recovery following disturbances. Cryopreservation may begin to help managers with this problem. If too few individuals spawn at a given time in a given area, we can freeze the sperm that is released for later use when fresh eggs become available. We can pool sperm from as many individual colonies as possible – locally, or even on a larger, regional scale – to artificially boost the diversity of crosses made when exposing frozen sperm to fresh eggs.
Researchers at the Hawai’i Institute of Marine Biology (Dr. Hagedorn’s team) set up Pocillopora meandrina colonies in anticipation of collecting their sperm for freezing. Photo credit: Liv Williamson
These promising methods have only been trialed on a few dozen stony coral species to date, with most frozen samples coming from Hawai’i and the Great Barrier Reef, meaning that they still have not been tested on hundreds of species from many regions. In the near future, it should be a priority for coral researchers and conservationists to attempt to freeze material from as many species and sites as possible, in order to quickly preserve the maximum amount of coral diversity as populations dwindle.
We can also prioritize freezing sperm from colonies that survive bleaching events or disease outbreaks; these gametes may possess a level of resistance to these stressors somewhere in their genes that could result in more resistant, “fit” offspring.
For instance, corals in the Persian Gulf survive some of the hottest temperatures in the oceans, often failing to bleach even at 34 – 36°C (significantly past the normal bleaching threshold). Although many of the species here are the same as those found throughout the Indo-Pacific, these corals have evolved some mechanism of tolerating extreme heat that are not seen in other regions. If we can successfully cryopreserve sperm from heat-tolerant Persian Gulf corals, we can transport and use it to inject heat-tolerance genes into new generations of at-risk populations of Indo-Pacific corals. In this way, assisted gene flow could soon become a powerful tool for rapidly propagating desirable traits within and among populations. With active intervention, cryopreservation of gametes may assist with creating diverse, viable, and even “climate-smart” offspring even while coral populations are increasingly unable to do so in nature.
A large colony of the endangered pillar coral Dendrogyra cylindrus near Abaco, the Bahamas. Photo credit: Liv Williamson
This technology may serve as a particularly crucial intervention for species on the brink of extinction. Dendrogyra cylindrus, the pillar coral, is a Caribbean reef-building species that is highly endangered throughout most of its historical range (Neely et al. 2018). It is highly susceptible to Stony Coral Tissue Loss Disease (SCTLD) and bleaching, and has experienced dramatic population declines in recent decades. With their high sensitivity to environmental stress, species like these may have a very hard time responding positively to other restoration initiatives like coral gardening. As such, in order to preserve what is left of this beautiful species, we should consider concentrating our cryopreservation efforts on genetically banking material from the most at-risk coral populations.
“Cryopreservation offers a wealth of potential for coral restoration, but needs to be applied more widely and trialed rapidly with as many species as possible. ”
Lobactis scutaria larvae under a microscope. Photo credit: Liv Williamson
Hagedorn and colleagues are currently developing methods to cryopreserve coral larvae. They have succeeded in doing so with the Indo-Pacific coral Lobactis scutaria (Daly et al. 2018), but have had trouble replicating the technique between species, years and even larval batches. While the sperm freezing and thawing protocols require minimal technical equipment and can be performed for relatively low cost, the existing larval thawing protocol requires a specific type of laser to warm the larvae at an extremely rapid rate (Daly et al. 2018). This equipment is very specialized and expensive, and thus this method may not be as readily scaled up and performed by scientists all over the world.
The next frontier for coral cryopreservation is freezing and thawing entire fragments of live coral tissue, a technique that members of the Hagedorn Lab are testing. Tissue fragments would be important resources for reviving entire genotypes of corals if, once thawed, they could continue to grow and form colonies once again.
“Microfragments” of various massive coral species in an offshore nursery near Key Biscayne, FL. Photo credit: Liv Williamson
Corals form important symbioses with single-celled algae called “Symbiodiniaceae.” Image credit: Scott Santos, Auburn University
Finally, cryopreservation techniques for Symbiodiniaceae, the algae that live in coral tissues and provide the majority of their energy for growth and calcification, remain elusive but hold promise for conservation. Most corals cannot survive for very long or grow to build reefs if they do not have a stable partnership with these algae, and in many cases this partnership is highly specific (Douglas 1998, Weis et al. 2001, Fabina et al. 2012).
Although Symbiodiniaceae are a very diverse family of algae (LaJeunesse et al. 2018) and most corals are capable of hosting a variety of types (Silverstein et al. 2012), some coral species almost exclusively associate with a single algal species. For instance, Dendrogyra cylindrus, the highly threatened Caribbean reef-builder mentioned above, only hosts Breviolum dendrogyrum (Chan et al. 2019). As such, if at some point in the future we intend to “revive” Dendrogyra by cryopreserving and eventually thawing it, we need to ensure we have its obligate algal partner ready to infect it with, otherwise it may not survive. Therefore, cryopreservation of a wide variety of Symbiodiniaceae types will be important to ensure that future coral populations have access to diverse algal populations in order to aid their calcification and reef building.
Dr. Chiahsin Lin and colleagues recently published the first report of successful cryopreservation and thawing of Symbiodiniaceae cells. They froze algal cells from the thermotolerant genus Durusdinium for 2 and 10 days in liquid nitrogen, then thawed and cultured them for 2 months with continued growth and proliferation. These are exciting results, and should be replicated for additional genera/species to begin genetically banking these important algae.
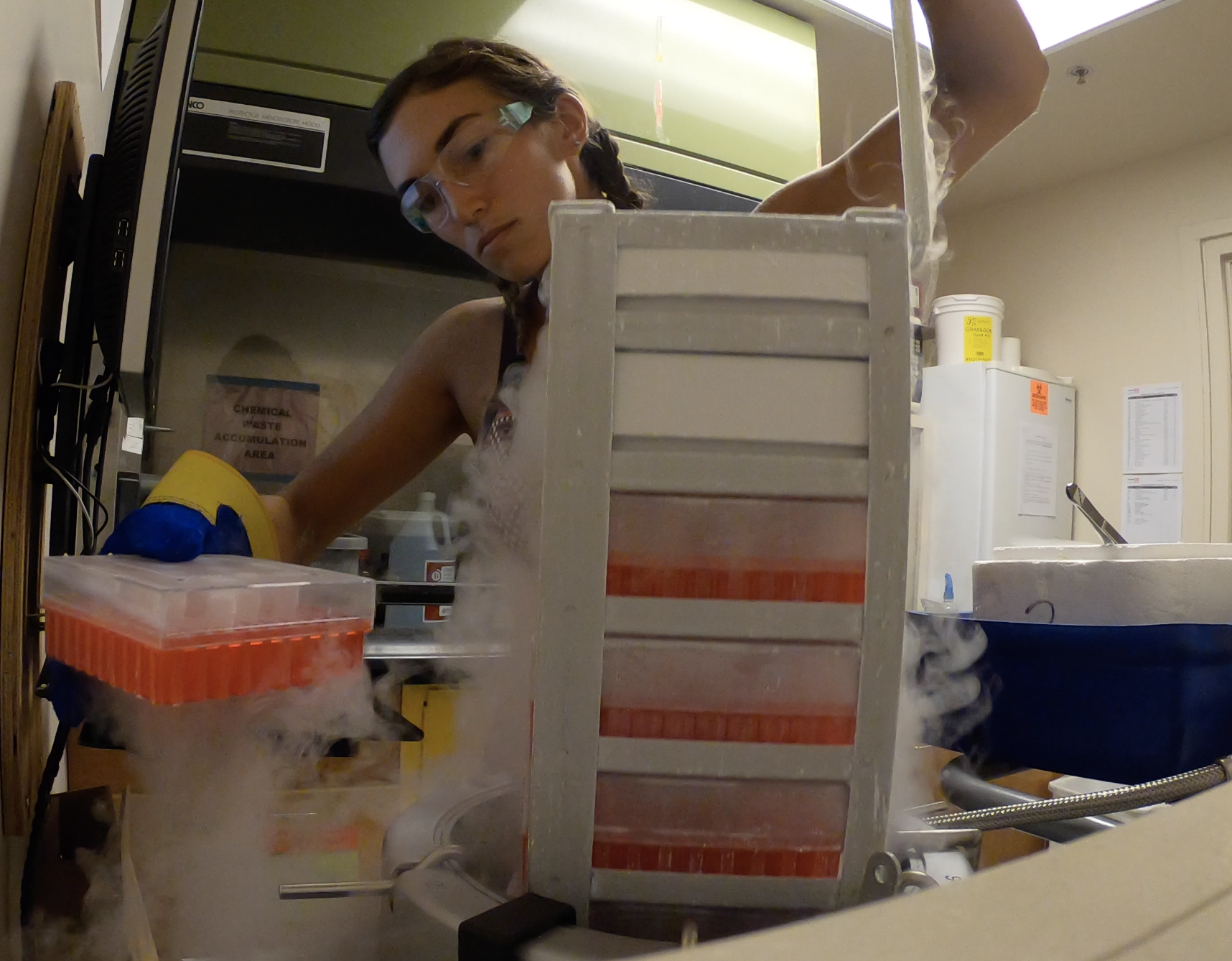
Ph.D. student Liv Williamson archives frozen coral sperm samples in liquid nitrogen. Photo credit: Liv Williamson
Conclusion
In summary, cryopreservation offers a wealth of potential for coral restoration, but needs to be applied more widely and trialed rapidly with as many species as possible. I personally believe in a “fast fail” approach, where any interested scientists with access to reproductive material can learn the basics of freezing and thawing sperm and try it whenever they can with the species they work with. Ideally, this will help to quickly build a body of knowledge, identify species that “work” given our current methods and species that require more research, and create a global community of stakeholders contributing material to genetic banks and generating knowledge about this novel mode of coral conservation.
All the best,
Liv